Protein synthesis with the ketoacid-hydroxylamine (KAHA) ligation
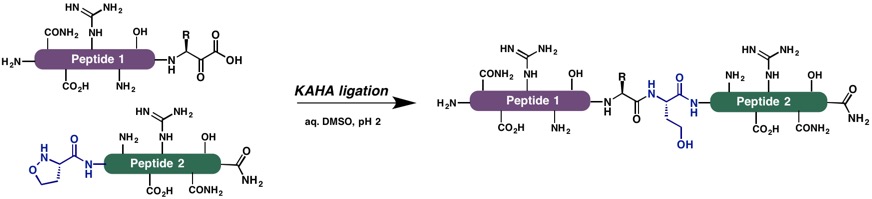
The ability to chemically synthesize biologically active proteins in a controlled fashion is one of the greatest achievements of synthetic chemistry in the last 20 years. As part of these efforts, our group reported the α-ketoacid–hydroxylamine (KAHA) ligation as a chemoselective coupling of large, unprotected peptide segments. The KAHA ligation employs C-terminal peptide α-ketoacids (KAs) and N-terminal peptide hydroxylamines (HAs), which react chemoselectively to form amides or esters. This reaction proceeds in aqueous media without reagents or catalysts.
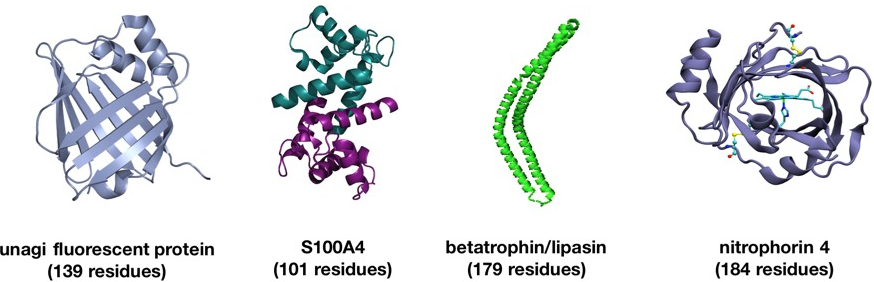
By developing new building blocks and chemical reactions, we can now easily prepare large peptide segments (40+ residues) bearing the necessary reaction partners – C-terminal peptide α-ketoacids and N-terminal peptide hydroxylamines – by standard Fmoc-SPPS. Along with innovative, orthogonal protecting group strategies, we can easily iterate segment ligations for the synthesis of protein targets up to about 200 residues. Recent examples of proteins prepared with the KAHA ligation include Pup, CspA, UFM1, SUMO2, SUMO3, S100A4, Nitrophorin 4 and betatrophin(lipasin).
Our ability to prepare synthetic proteins with any kind of unnatural amino acids, post-translational modification, fluorescent tag, or synthetic handle forms the basis of our increasing efforts in chemical biology and the synthesis of synthetic proteins of therapeutic interest.
Selected publications:
(6) Bode, J. W.; Fox, R. M.; Baucom, K. D. external page"Decarboxylative Condensations of alpha-Ketoacids and N-Alkylhydroxylamines: A New Amide Ligation Reaction"call_made Angew. Chem. Int. Ed. 2006, 45, 1248–1252.
(17) Ju, L.; Lippert, A. R.; Bode, J. W. external page"Stereoretentive Synthesis and Chemoselective Amide-Forming Ligations of C-Terminal Peptide alpha-Ketoacids"call_made J. Am. Chem. Soc. 2008, 130, 4253–4255.
(23) Ju, L.; Bode, J. W. external page"A general strategy for the preparation of C-terminal peptide a-ketoacids by solid phase peptide synthesis"call_made Org. Biomol. Chem. 2009, 7, 2259–2264.
(49) Wu, J.; J. Ruiz-Rodríguez; Comstock, J. M.; Dong, J. Z.; Bode, J. W. external page"call_madeexternal pageSynthesis of human GLP-1 (7–36) by chemoselective α-ketoacid–hydroxylamine peptide ligation of unprotected fragments"call_made Chem. Sci. 2011, 2, 1976–1979.
(54) Review article: Pattabiraman, V.; Bode, J. W. external page“Rethinking Amide Bond Synthesis”call_made Nature 2011, 480, 471–479.
(62) Pattabiraman, V. R.; Ogunkoya, A. O.; Bode, J. W. external page"Chemical Protein Synthesis by Chemoselective α-Ketoacid–Hydroxylamine (KAHA) Ligations with 5-Oxaproline"call_made Angew. Chem. Int. Ed. 2012, 51, 5114–5118.
(72) Ogunkoya, A. O.; Pattabiraman, V. R.; Bode, J. W. external page"Sequential α-Ketoacid-Hydroxylamine (KAHA) Ligations: Synthesis of C-Terminal Variants of the Modifier Protein UFM1"call_made Angew. Chem. Int. Ed. 2012, 51, 9693–9697.
(98) Wucherpfennig, T. G.; Rohrbacher, F.; Pattabiraman, V. R.; Bode, J. W. external page"Formation and Rearrangement of Homoserine Depsipeptides and Depsiproteins in the α-Ketoacid–Hydroxylamine Ligation with 5-Oxaproline"call_made Angew. Chem. Int. Ed. 2014, 53, 12244–12247.
(99) Wucherpfennig, T. G.; Pattabiraman, V. R.; Limberg, F. R. P.; Ruiz-Rodríguez, J.; Bode, J. W. external page"Traceless Preparation of C-Terminal α-Ketoacids for Chemical Protein Synthesis by α-Ketoacid–Hydroxylamine Ligation: Synthesis of SUMO2/3"call_made Angew. Chem. Int. Ed. 2014, 53, 12248–12252.
(102) Murar, C. E.; Thuaud, F.; Bode, J. W. external page"KAHA Ligations that form Aspartyl Aldehyde Residues as Synthetic Handles for Protein Modification and Purification"call_made J. Am. Chem. Soc. 2014, 136, 18140–18148.
(110) Rohrbacher, F.; Deniau, G.; Luther, A.; Bode, J. W. external page"Spontaneous Head-to-Tail Cyclization of Unprotected Linear Peptides with the KAHA Ligation"call_made Chem. Sci. 2015, 6, 4889–4896.
(115) He, C.; Kulkarni, S. S.; Thuaud, F.; Bode, J. W. external page"Chemical Synthesis of the 20 kDa Heme Protein Nitrophorin 4 by α-Ketoacid-Hydroxylamine (KAHA) Ligation"call_made Angew. Chem. Int. Ed. 2015, 54, 12996–13001.
(121) Harmand, T. J.; Murar, C. E.; Bode, J. W.; external page"Protein chemical synthesis by α-ketoacid-hydroxylamine ligation"call_made Nature Protocols 2016, 11, 1130-1147
(122) Thuaud, F.; Rohrbacher, F.; Zwicky, A.; Bode, J. W.; external page"Incorporation of Acid-Labile Masking Groups for the Traceless Synthesis of C-Terminal Peptide α-Ketoacids"call_made Org. Lett. 2016, 18, 3670–3673
(137) Rohrbacher, F.; Zwicky, A.; Bode, J. W. external page"Chemical Synthesis of a Homoserine-Mutant of the Antibacterial, Head-to-Tail Cyclized Protein AS-48 by α-Ketoacid–Hydroxylamine (KAHA) Ligation"call_made Chem. Sci. 2017, 8, 4051–4055
(141) Harmand, T. J.; Pattabiraman, V. R.; Bode, J. W. external page"Chemical Synthesis of the Highly Hydrophobic Antiviral Membrane Associated Protein IFITM3 and Modified Variants"call_made Angew. Chem. Int. Ed. 2017, 56, 12639–12643
(142) Review article: Bode, J. W. external page"Chemical Protein Synthesis with the α-Ketoacid–Hydroxylamine Ligation"call_made Acc. Chem. Res. 2017, 50, 2104–2115