Catalytic, enantioselective annulations with chiral N-heterocyclic carbenes
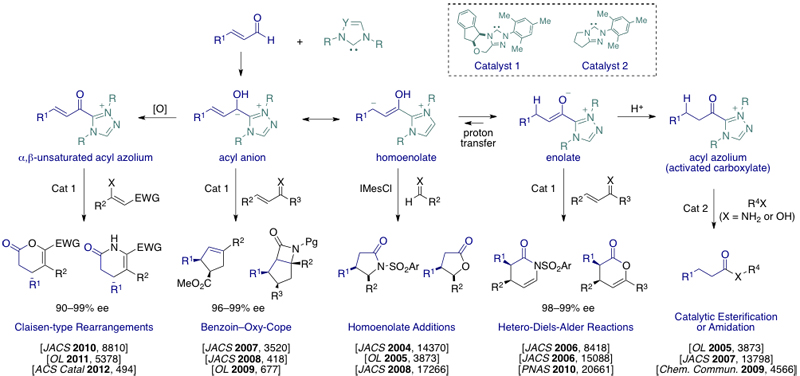
Previous efforts from our group led to an new branch of catalytic asymmetric synthesis, commonly known as “Chiral N-heterocyclic carbene (NHC) catalysis”. We pioneered the catalytic generation of new reactive species by the combination of N-heterocyclic carbenes and α-functionalized aldehydes. Since these first reports, we have expanded the NHC-catalyzed generation of reactive intermediates to the formation of four novel and distinct reactive intermediates: i) homoenolate equivalents, ii) enolate equivalents, iii) acyl azoliums, which serve as activated carboxylic acids, and iv) α,β-unsaturated acyl azoliums. Nearly all of these reactions are promoted by N-Mesityl substituted triazolium salts (1-2) developed in our group and currently sold by external pageAldrichcall_made, external pageTCIcall_made, and external pageBioblockscall_made. Examples from our group are shown below.
We are no longer pursuing NHC-catalyzed carbon–carbon bond formation in our group. Many other groups continue this research, which remains a fascinating and productive area of asymmetric catalysis.